Home » Science & Technology
The Encapsulated Cell-Based Therapy (ECT) Drug Delivery Platform
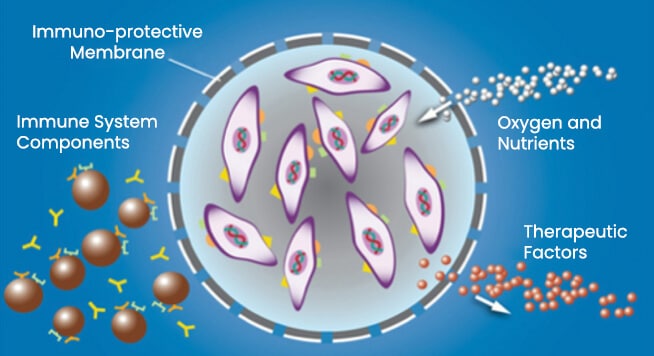
- ECT is a novel cell-based drug delivery therapy platform designed to continually produce and deliver therapeutic proteins to the retina.
- The ECT drug delivery platform consists of a human retinal cell line, encapsulated within a semi-permeable membrane that allows nutrients to diffuse in, and therapeutic proteins to diffuse out.
- The cells in the ECT drug delivery platform can be genetically engineered to produce therapeutic proteins.
Intraocular Drug Delivery
- With our ophthalmic focus, the ECT capsule used in the Encapsulated Cell-Therapy Drug Delivery Platform is placed into the vitreous and has the potential to consistently deliver therapeutic proteins for greater than two-years following a single application (Kauper et al., 2012).
- The Encapsulated Cell-Based Therapy Drug Delivery Platform helps to potentially mitigate the risks associated with repeated intra-ocular or sub-retinal injections.
- Use of the ECT Delivery Platform can create an immune-protective microenvironment.
Ciliary Neurotrophic Factor (CNTF)
- CNTF is a well-studied protein that has been shown to be protective of structures in the retina including photoreceptors and ganglion cells.
- As shown in a preclinical model of the photoreceptor degeneration, the eyes treated with CNTF have a higher number of photoreceptors, while the non-treated diseased eyes progressively lost photoreceptors.
